Describe the Bohr Model of the Atom
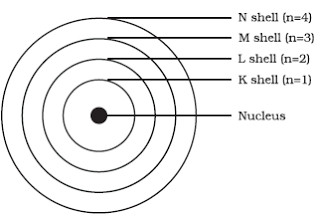
According to Bohr Atomic model a small positively charged nucleus is surrounded by revolving negatively charged electrons in fixed orbits. The Bohr model named after Danish physicist Niels Bohr of an atom has a small positively charged central nucleus and electrons orbiting in at specific fixed distances from the nucleus.

Bohr Model Description Development Britannica
This model is known as Bohrs model.

. In 1913 Niels Bohr proposed the atomic Hydrogen model. This model provides especially the solution to the problem of the failure of classical. The atom was described as a positively-charged sphere embedded with electrons.
The single electron in hydrogen revolves around the nucleus in one of a limited number of circular orbits. The Bohr model of the hydrogen atom explains the connection between the quantization of photons and the quantized emission from atoms. Bohr model description of the structure of atoms especially that of hydrogen proposed 1913 by the Danish physicist Niels Bohr.
Ii There are only certain special orbits known as discrete orbits in which the electrons revolve around the nucleus are known to be present inside the atom. A transition from a higher orbit to a lower orbit will releasequantized energies of light which would explain the light spectrum. Bohr incorporated Plancks and Einsteins quantization ideas into a model of the hydrogen atom that resolved the paradox of atom stability and discrete spectra.
The Modern Atomic Model Today The current model of the atom shows an atom that is mostly empty space. 4 While rotating in discrete circles the electrons dont transmit energy. Bohrs model consists of a small nucleus positively charged surrounded by negative electrons moving around the nucleus in orbits where he found out that an electron located away from the nucleus has more energy as compared to electrons close to the nucleus.
In this model the electrons travel around the nucleus of an atom in distinct circular orbits or shells. The Bohr Model of the Hydrogen Atom tries to fill in some of the holes left by Rutherfords model. The Bohr model of the atom a radical departure from earlier classical descriptions was the first that incorporated quantum theory and was the predecessor of wholly quantum-mechanical models.
2 An atoms energy does not change while the electron moves in a particular orbit. The model was proposed by physicist Niels Bohr in 1913. In atomic physics the Bohr model if the atom also known as the Rutherford-Bohr model is modern model of the hydrogen atom introduced by Danish physicist Niels Bohr working with Ernest Rutherford at the University of Manchester in 1913.
Electrons revolve around the nucleus. According to Bohr model the revolving paths traced by electrons are called orbits or shells. The Bohr Model is a structural model of an atom.
Ernest Rutherford model- Nuclear model of an atom Neil Bohrs model of the atom- Planetary model Erwin Schrödingers model-Quantum model. What is the most accurate atomic model. This is the modern atom model.
Bohr model is utilized for giving a lucid explanation to the features of an atom. Bohrs Model Of An Atom 1An atom is made up of three particleselectronsprotons and neutronsElectrons have negative chargeprotons have positive charge whereas neutrons have no chargeDue the presence of equal number of negative electrons and positive protonsthe atom on the whole is electrically neutral. Bohr described the hydrogen atom in terms of an electron moving in a circular.
Electrons revolve around the nucleus of an atom in fixed paths called orbits or shells. The Bohr Model of The Atom 1 The H atom only has certain energy levels they are determined by fixed circular orbits of electrons around the nucleus. The most accurate atomic model was given by Neil Bohr and is called Bohrs model of atom or Planetary model.
Bohrs Model of an atom. The model is also referred to as the planetary model of an atom. The model proposes that the maximum number of electrons that can be accommodated in any particular orbit is 2n 2 where n is the number of orbits.
First published in 1807 many of Daltons hypotheses about the microscopic features of matter are still valid in modern atomic theory. 3 Certain exceptional circles known as discrete circles of electrons are permitted inside the molecule. A model proposed by Niels Bohr to support his hypothesis about electronsin a hydrogen atom.
Describe Bohrs model of the atom. Bohr Model of the Atom. The Bohr model is used to account for the spectrum of the hydrogen atom but the basic idea is the same for all elements.
2 Electrons rotate around the nucleus. The Bohr model and all of its successors describe the. I Atoms has nucleus that is present in the centre.
Neil Bohr put forward the following postulates about the model of an atom. Bohr proposed that electrons do not radiate energy as they orbit the nucleus but exist in states of constant energy which he called stationary states. Bohrs Model is an atomic model proposed by a Danish Physicist Niels Bohr in 1913.
Laminiaduo7 and 6 more users found this answer helpful. Following the discoveries of hydrogen emission spectra and the photoelectric effect the Danish physicist Niels Bohr 18851962 proposed a new model of the atom in 1915. 5 These circles or shells are called energy levels.
Main ideas of the Bohr model. Describe the Bohr model of the hydrogen atom Use the Rydberg equation to calculate energies of light emitted or absorbed by hydrogen atoms Following the work of Ernest Rutherford and his colleagues in the early twentieth century the picture of atoms consisting of tiny dense nuclei surrounded by lighter and even tinier electrons continually. This is a model of the atomic structure in which electronstravel around the nucleus in well-defined orbits determined by quantumconditions.
According to this model In an atom the electrons revolve around the nucleus in definite energy levels called orbitsshells. Bohrs model of the molecule 1 Atoms have a nucleus in the middle. He concluded that electron will have more energy if it is located away from the nucleus whereas the electrons will have less energy if.
It has a special place in history because it introduced quantum theory which gave rise to quantum mechanics.

The Bohr Model Introduction To Chemistry

Describe Bohr S Model Of The Atom Snapsolve

Bohr S Model Of An Atom With Postulates And Limitations Of Bohr S Model Bohr Model Applicable To
No comments for "Describe the Bohr Model of the Atom"
Post a Comment